MyopiaX - Clinical study on targeted light biomodulation for progressive myopia
Dopavision, a pioneer in the advancement of pediatric ophthalmology, announced the first results of its MyopiaX-1 clinical trial (NCT04967287) on June 3. The 6-month results of the randomized, controlled trial demonstrate the safety and tolerability of MyopiaX.

The results add to the growing body of scientific evidence confirming the mechanism of action of MyopiaX and its potential therapeutic suitability for slowing the progression of myopia.
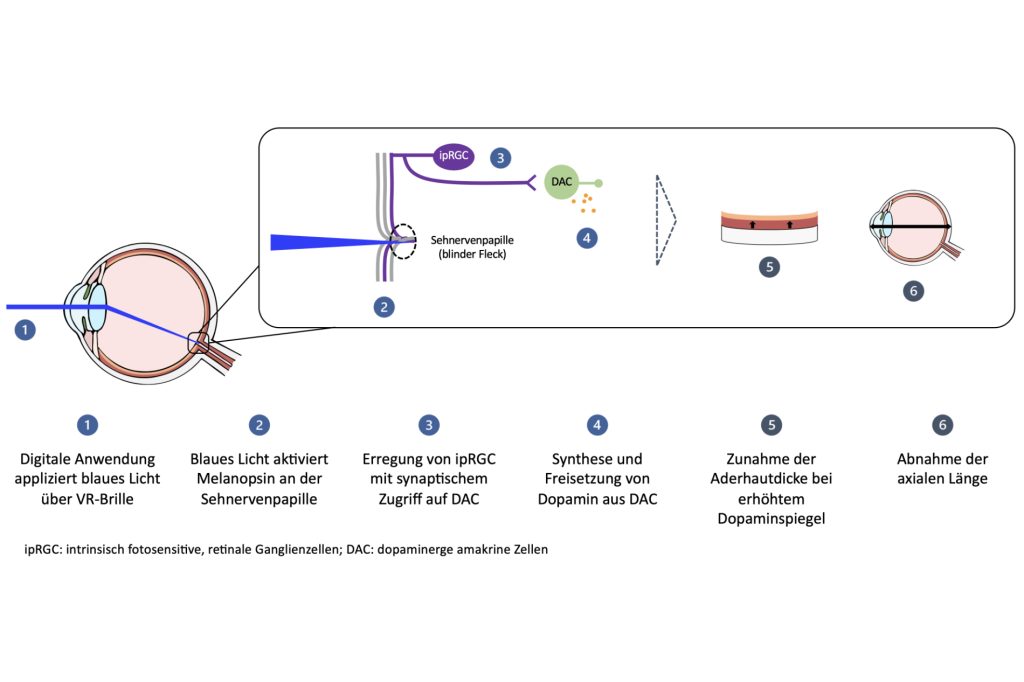
MyopiaX works by targeting light biomodulation to the eye with the aim of limiting the progression of myopia in children. It is the first targeted intervention of its kind that uses light from a smartphone app to non-invasively stimulate targeted photoreceptors in the eye, regulating innate dopamine signaling pathways and slowing the progression of myopia. MyopiaX is applied via an intuitive and child-friendly digital platform that seamlessly integrates myopia treatment into everyday life using consumer electronics such as smartphones. This non-invasive, globally accessible approach aims to tackle the root cause of the growing global problem of myopia. According to forecasts, almost half of the world's population will be short-sighted by 2050.
In the study conducted in six European countries, 124 short-sighted children were randomly assigned in a 2:1 ratio to either MyopiaX or a control group. Over a six-month period, treatment with MyopiaX showed signs of a clinical effect on the rate of progression of myopia. MyopiaX proved to be safe and tolerable during the six-month trial period and no adverse events were reported, confirming that MyopiaX is a low-risk procedure.
"The six-month results represent a significant step forward in the clinical development of MyopiaX," said Prof. Dr. Ian Flitcroft, Fellow of the Royal College of Ophthalmologists and principal investigator of the MyopiaX-1 study. "The results are an important addition to the existing evidence for the mechanism of action of MyopiaX light stimulation."
Dr. Mark Wuttke, CEO of Dopavision, adds: "The initial results of the MyopiaX-1 study after six months are a great success for Dopavision. Our goal with MyopiaX is to improve the treatment of millions of children worldwide by providing them with a safe, non-invasive and responsive alternative that helps young people build a healthier and brighter future." The ongoing MyopiaX-1 clinical trial is expected to be completed in September 2024. Dopavision is currently in discussions with regulatory authorities to clarify the necessary steps for further development and approval of MyopiaX.
Further information is available here: www.dopavision.com